Crystal Morphology in an Iron-Sulfide-Containing Protein
Crystal Morphology in an Iron-Sulfide-Containing Protein
Submitted by Daniel Dowling and Catherine Drennan of the Drennan Lab in the Department of Chemistry at MIT
MIT Department of Biology, MIT Department of Chemistry
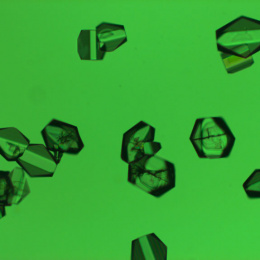
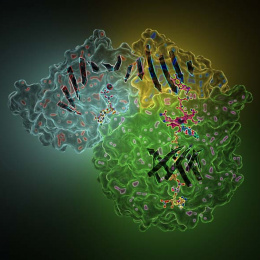
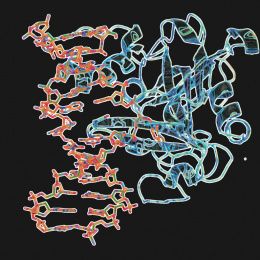
"Displayed are crystals of a protein involved in the biosynthesis of a family of deazapurine natural products, which include the antibiotic toyocomycin and the tRNA modified base queuosine. Using these crystals, we were able to determine the molecular structure of this protein with all components necessary for activity. This includes an iron-sulfur cluster, which is why the crystals are brown. The X-ray crystal structure obtained from these crystals revealed important structural interactions for binding product."