Helical Nanostructures in Amyloid-Beta Proteins 4
Helical Nanostructures in Amyloid-Beta Proteins 4
Zhuyu Peng
McGovern Institute for Brain Research, Picower Institute for Learning and Memory
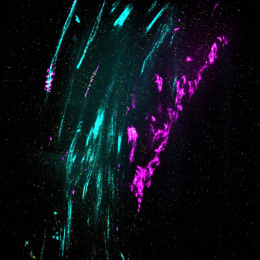
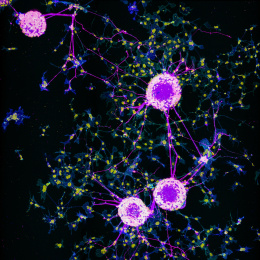
This image shows 800-times-magnified views of a helical amyloid-beta nanostructure (in color magenta) spirally tangling around a bundle of axons (in color cyan) in an Alzheimer’s Disease (AD) mouse model 5xFAD. Interestingly, clusters of sodium ion channels (in yellow) were found co-localizing with these distinct amyloid-beta nanostructures, suggesting intriguing functional relationships between these proteins and potential functional consequences.
Although the misfolding of amyloid-beta (Aβ) in the forms of Aβ plaques is a prominent pathological hallmark of Alzheimer’s Disease (AD), the roles of which remains investigated partially because Aβ plaques is only weakly correlated with the degree of cognitive decline in AD. However, it’s shown that different forms of soluble and insoluble Aβ aggregates including Aβ oligomers, Aβ fibrils etc, may be associated with different disease stages. Therefore, understanding the structural and interaction details of Aβ aggregates could provide mechanistic insights into how different conformations of Aβ induce neurotoxicity.
This DNA-probe-based super-resolution microscopy not only enables previously unseen structural features of Aβ helical nanostructure, it also reveals interaction and position details of this protein.