Enhanced Mucosal Delivery to Improve Immune Protection 2
Enhanced Mucosal Delivery to Improve Immune Protection 2
Brittany Hartwell, Jason Chang
Koch Institute at MIT
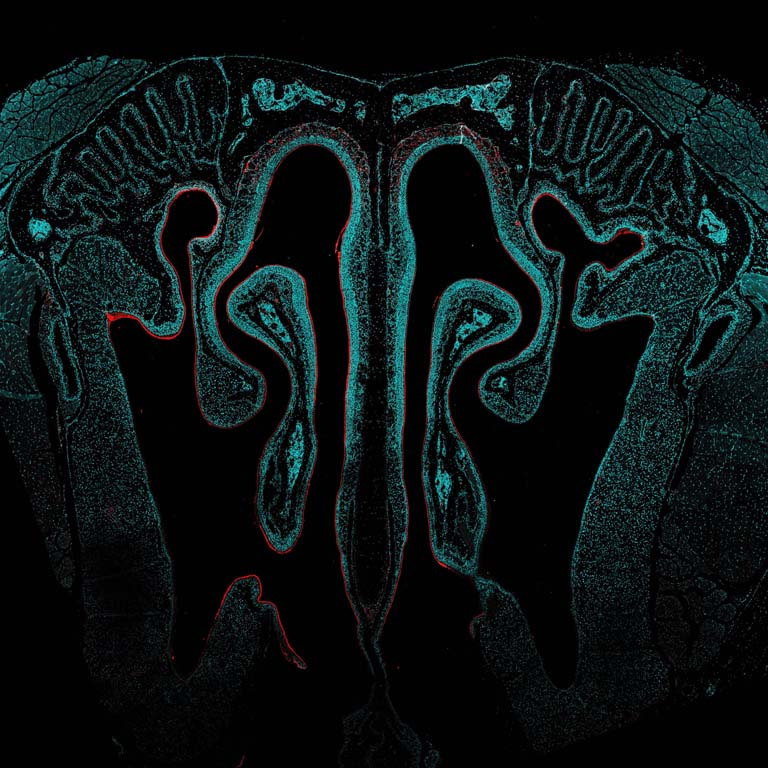
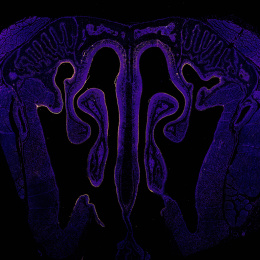
This image shows a cross-section of the nasal passage from a mouse that received an intranasal vaccine developed in the Irvine Lab. The vaccine was designed to be better at transporting into and across the mucosal tissues in the nose, in order to be taken up into the underlying nasal-associated immune tissues in greater amounts to activate a stronger immune response. Compared to injected vaccines, mucosal vaccines (like those administered intranasally) are better at activating a mucosal immune response, which is important for establishing a ‘frontline defense’ of immune protection at mucosal tissues where many infectious diseases are transmitted, such as HIV and SARS-CoV-2. However, mucosal vaccines have historically been plagued by delivery challenges and poor uptake leading to weak immune protection. In this case, our vaccine was engineered to overcome mucosal barriers and evade rapid clearance mechanisms by ‘hitchhiking’ on a chaperone protein found naturally in the mucosal environment. Here, we show that our vaccine is indeed accomplishing this: rather than being rapidly cleared (which is what we observe with a control non-engineered version of this vaccine), our engineered vaccine is sticking around in the nasal cavity for multiple days after administration, lining the nasal tissue and being taken up into the underlying cells.
The vaccine was labeled with a fluorescent molecule so it could be visualized by microscopy. In these images, the signal of the vaccine (in red) can be seen lining the textured nasal tissue (cyan) against the intricate architecture of the nasal cavity (background and negative space).